The production and processing technology of making pockets
Medical-Grade Ostomy Pouches: Clinical Applications and Advanced Manufacturing Technologies
As critical medical devices in modern healthcare, ostomy pouches have become indispensable in clinical management. This technical white paper elucidates their clinical specifications and details the engineering advantages of their primary manufacturing equipment - high-frequency (HF) welding systems.
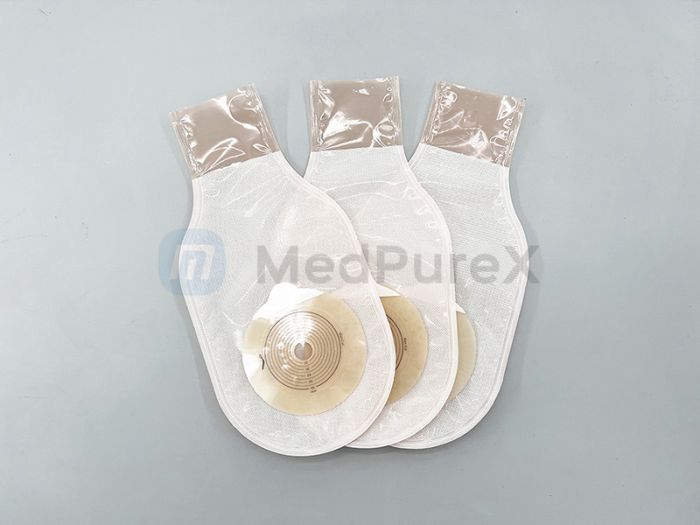
Section I: Ostomy Pouch Technical Profile
Definition & Clinical Utility
Ostomy pouches (medical containment devices) are engineered to securely collect and contain bodily waste (urine/fecal matter) for patients with excretory dysfunction caused by:
• Traumatic brain injuries (TBI)
• Spinal cord injuries (ASIA Grade A-C)
• Neurogenic bladder/bowel disorders
• Post-surgical recovery states
Material Science Specifications
Constructed from ISO 10993-certified polymers:
- Polypropylene (PP) matrices (0.5-1.2mm thickness)
- Polyester fiber reinforcement (120-200 denier)
Key material properties:
✓ Biocompatibility (Class VI compliance)
✓ Hydrolysis resistance (85% RH, 70°C accelerated aging)
✓ Tensile strength >15 MPa (ASTM D882)
Customization capabilities include:
• Anatomical contouring (15°-45° curvature radii)
• Capacity gradients (200-1000 mL)
• Sterile barrier configurations
Section II: Precision Manufacturing with HF Welding Systems
5 Key Technological Superiorities
1. Energy-Efficient Processing
- Achieves 40-60% energy savings vs conventional thermal welding
- Rapid heating cycles: 2-8 sec weld times (20-40kW RF generators)
- Power factor >0.95 (IEC 61000-3-2 compliant)
2. Microprocessor-Controlled Precision
- Closed-loop PID regulation (±0.5% power stability)
- Dynamic frequency tracking (27.12±0.6MHz)
- Weld uniformity: <5% thickness variation (per EN 28510-2)
3. Intuitive HMI Operation
- 7" Industrial Touchscreen (IP65 rated)
- 100-program memory with cloud backup
- Multi-stage safety interlocks:
○ Over-temperature shutdown (90°C threshold)
○ Arc detection <2μs response
○ Pressure monitoring (0-1.5MPa range)
4. Multi-Domain Applicability
Medical device welding applications:
- Drainage bags (3-side seal patterns)
- IV solution containers (luer port integration)
- Surgical drapes (breathable seam welding)
Seal performance metrics:
◆ Burst pressure >60kPa (ISO 11607)
◆ Helium leak rate <1×10⁻⁶ mbar·L/s
5. Eco-Conscious Manufacturing
- Zero VOC emissions (EPA Method 25 compliant)
- 98% energy conversion efficiency
- REACH-compliant material handling

.jpg)
