The production process flow of sterile isolation protective sleeves for TPU surgical robots
The production process flow of the sterile isolation protective cover for TPU surgical robots mainly includes the following steps:
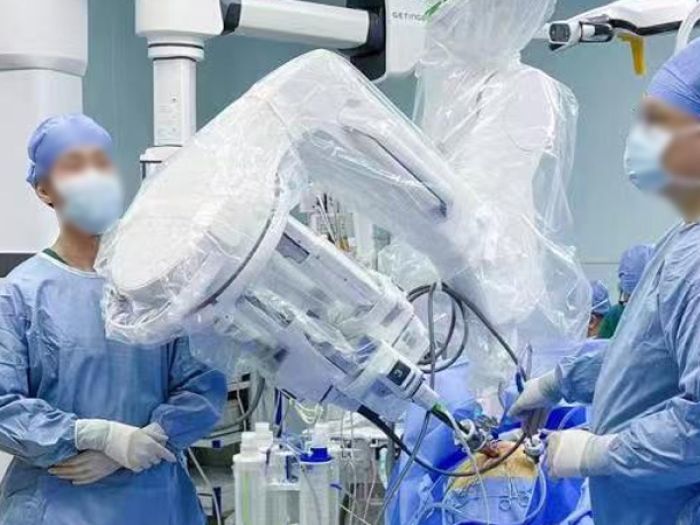
-
Material Selection and Preparation
- Select TPU materials that meet medical-grade standards, ensuring excellent biocompatibility, abrasion resistance, tear resistance, and high-temperature resistance.
- Conduct rigorous quality inspections to confirm compliance with relevant standards.
- Prepare TPU materials in required specifications and thicknesses based on design drawings, with necessary pretreatment (e.g., cleaning, drying).
-
Design
- Create design drawings tailored to the surgical robot's specific model and dimensions, defining the protective cover's size, shape, opening positions, and welding areas.
-
Cutting and Molding
- Use precision cutting equipment (e.g., laser cutting or mold forming) to shape TPU materials into required dimensions and profiles.
-
Preheating
- Preheat thermal welding equipment to an appropriate temperature to ensure optimal TPU material melting.
-
Positioning and Clamping
- Accurately position and clamp TPU components on the welding equipment to ensure tight contact between welding surfaces.
-
Heating and Welding
- Activate thermal welding equipment to heat and bond TPU materials. Adjust heating time and temperature based on material thickness to ensure weld seam strength, smoothness, and airtightness.
- Install safety mechanisms (e.g., anti-spark devices and spark suppressors) to protect high-frequency molds and machinery.
-
Cooling and Curing
- Place welded components on a cooling station to solidify and strengthen the bonds.
-
Assembly
- Assemble welded sections of the sterile protective cover, ensuring correct connections and design compliance.
-
Inspection
- Conduct comprehensive quality checks, including visual inspection, weld seam integrity, and dimensional accuracy, to verify defect-free products that meet standards.
-
Sterilization
- Perform medical-grade sterilization (e.g., ethylene oxide sterilization) to achieve sterility. Validate sterilization efficacy to ensure compliance.
-
Packaging
- Package sterilized protective covers under aseptic conditions to prevent contamination during storage and transportation.
-
Storage
- Store packaged products in a cool, ventilated, and dry environment, avoiding moisture and direct sunlight. Monitor shelf life and storage conditions to ensure usability within validity periods.



.jpg)